Sprycel
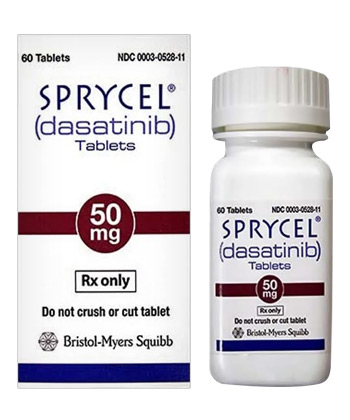
Sprycel
- In our pharmacy, you can buy Sprycel without a prescription, with delivery in 5–14 days throughout Australia. Discreet and anonymous packaging.
- Sprycel is used for the treatment of Ph+ chronic myeloid leukemia (CML) and Ph+ acute lymphoblastic leukemia (ALL). The drug works as a protein kinase inhibitor, targeting kinases to inhibit cancer cell growth.
- The usual dose of Sprycel varies by condition but can be 100 mg once daily for adult Ph+ CML patients.
- The form of administration is a tablet.
- The effect of the medication begins within a few hours after ingestion.
- The duration of action can last for 24 hours.
- Do not consume alcohol while taking Sprycel.
- The most common side effects include myelosuppression, fatigue, and diarrhea.
- Would you like to try Sprycel without a prescription?
Basic Sprycel Information
• INN (International Nonproprietary Name): Dasatinib
• Brand Names Available in Australia: Sprycel
• ATC Code: L01EA02
• Forms & Dosages: Tablets (20 mg, 50 mg, 70 mg, 80 mg, 100 mg, 140 mg)
• Manufacturers in Australia: Bristol-Myers Squibb
• Registration Status in Australia: Prescription only
• OTC / Rx Classification: Rx
Everyday Use & Best Practices
Understanding how to best incorporate Sprycel into daily routines is key to ensuring its effectiveness. Consistency is fundamental, as taking the medication at the same time each day can help maintain stable blood levels. For many Australians, this can mean fitting the dosing around their lifestyle.
Morning vs Evening Dosing
Choosing whether to take Sprycel in the morning or evening may depend on personal schedules. Many prefer taking it in the morning, as this can help incorporate it into the beginning of their day. This might be beneficial for those who take other medications or supplements in the morning, creating a routine. Alternatively, taking Sprycel in the evening can be more suitable for individuals with busy mornings, ensuring they don’t forget a dose. Regardless of the choice, the focus should be on consistency.
Taking With or Without Meals
Dosage can be taken either with or without food, which provides flexibility in daily scheduling. However, taking it with food can sometimes help mitigate any gastrointestinal discomfort that may arise. To maximise absorption, it’s advisable to consult with the prescribing health professional regarding the best method for individual situations. In any case, maintaining regularity is the secret to effective management with Sprycel.
Safety Priorities
Safety is paramount when using Sprycel. Awareness of who should refrain from using it and understanding its associated risks can aid in effective patient management. Consulting with healthcare professionals ensures that those with specific factors are monitored appropriately.
Who Should Avoid It
Certain populations should avoid Sprycel due to safety concerns. This includes individuals with known hypersensitivity to dasatinib or any of the tablet's excipients. According to TGA guidelines, patients with severe allergic reactions or those experiencing significant heart issues should be particularly cautious. Individuals with liver impairments or blood platelet disorders also require close monitoring if prescribed this medication. Always consult a healthcare provider to determine if Sprycel is appropriate.
Activities to Limit
While on Sprycel, be mindful that it can affect alertness and physical coordination. This means individuals may need to limit activities that require high concentration, such as driving or operating heavy machinery. It's crucial to consider these effects, ensuring personal safety and workplace safety while undergoing treatment.
Dosage & Adjustments
Getting the dosage right is essential for maximising the benefits of Sprycel. Understanding the prescribed dosage and when adjustments may be necessary can help in managing health conditions effectively.
General Regimen
The standard dosage for adults with chronic myeloid leukaemia (CML) typically starts at 100 mg once daily, while specific adjustments are made for children based on body surface area. For persistent cases, dosages may rise to 140 mg daily depending on clinical response. It’s critical to adhere to the prescription details outlined by your healthcare provider.
Special Cases
For elderly patients or individuals with additional health concerns, monitoring and possible adjustments are essential. Those with concurrent health issues may find themselves needing a tailored approach to Sprycel. Despite these considerations, the standard adult dosing of 100 mg is often recommended as a starting point, ensuring effective management across various demographics.
User Testimonials
Hearing from others can provide insights into the effectiveness of Sprycel and how it integrates into daily lives. Positive reports often highlight successful management of CML with minimal side effects.
Positive Reports from Australian Patients
Many Australians find that Sprycel has significantly enhanced their quality of life. Testimonials frequently mention how the drug has successfully stabilised their condition with manageable side effects. These positive experiences serve as a reminder of the importance of sticking to the prescribed regimen for optimal outcomes.
Common Challenges
Not all experiences are without hurdles. Some patients mention side effects ranging from fatigue to mild gastrointestinal discomfort, which can impact adherence to their treatment plan. Engaging with forums and platforms like ProductReview can shed light on these challenges and offer supportive community insights.
Buying Guide
Purchasing Sprycel in Australia can seem daunting, but several reliable sources make it easier. Whether through major pharmacy chains or local chemists, knowing where to go is essential. Patients are encouraged to verify pharmacy sources within their locality for availability.
Pharmacy Sources
- Chemist Warehouse
- Priceline
- TerryWhite Chemmart
These major pharmacies commonly stock Sprycel, making access straightforward for those with prescriptions.
Price Comparison
Cost can vary significantly when purchasing through the Pharmaceutical Benefits Scheme (PBS) compared to private payment. Patients should consider the benefits of PBS for reduced treatment costs, ensuring affordable access to their necessary medications. Checking with your pharmacy for the latest pricing and reimbursement options will provide clarity.
Formulation of Sprycel
Sprycel, known internationally as dasatinib, is manufactured by Bristol-Myers Squibb, marketed as E.R. Squibb & Sons, L.L.C. in the United States. Available primarily in tablet form, it comes in various strengths, including 20 mg, 50 mg, 70 mg, 80 mg, 100 mg, and 140 mg. Each strength is typically dispensed in bottles equipped with child-resistant caps, ensuring safety during storage. Notably, no injectable solution or cream formulations are marketed, making tablets the sole delivery method.
Key formulations of Sprycel:
- 20 mg tablets: Supplied in bottles of 60
- 50 mg tablets: Also in bottles of 60
- Other strengths (80 mg, 100 mg, 140 mg): Available in bottles of 30 or 60
This medication is classified under ATC code L01EA02, which targets protein kinases to inhibit the growth of cancer cells effectively. Since its approval, Sprycel has been pivotal in treating specific forms of leukaemia.
Approved Medical Indications for Sprycel
Approved since 2006 by the US FDA, Sprycel is indicated for the treatment of Ph+ chronic myeloid leukaemia (CML) and Ph+ acute lymphoblastic leukaemia (ALL) in both adults and children aged one year and above. The European Medicines Agency (EMA) mirrors these indications, including extensions for paediatric use. Such approvals underscore its global significance in managing these cancers.
Standard dosages vary based on the condition:
- For newly diagnosed Ph+ CML: 100 mg once daily for adults; 60 mg/m² for children.
- Ph+ ALL (resistant to prior treatment): 140 mg once daily for adults; in combination therapy for children at 60 mg/m² daily.
Additionally, the medication is part of the WHO List of Essential Medicines, further validating its role in standard cancer treatment protocols worldwide.
Warnings for Food and Drug Interactions
Careful consideration is required regarding potential interactions with food and other medications when using Sprycel. While it primarily affects cancer cells, its impact on healthy cells and processes can lead to various side effects.
Noteworthy warnings include:
- **QT interval prolongation or cardiac arrhythmias:** Regular monitoring is essential, especially if the patient has pre-existing conditions.
- **Uncontrolled hypertension:** Elevated blood pressure could exacerbate side effects.
- **Bleeding disorders or liver impairment:** Close monitoring is advisable due to increased risks.
- **Use in patients on anticoagulants:** Increases the likelihood of bleeding complications.
Patients should be advised to consult with healthcare providers regarding dietary habits and any medications they are taking to minimise risks effectively.
Latest Research Evidence on Sprycel
Ongoing research continues to bolster the efficacy and safety profiles of Sprycel in various treatment scenarios. Studies highlight its effectiveness in patients resistant to other therapies, showcasing dasatinib's unique ability to target Bcr-Abl and SRC family kinases.
Key research findings include:
- Studies indicate improved survival rates in patients with resistant CML and ALL when Sprycel is used.
- Meta-analyses report the drug’s efficacy in managing side effects commonly associated with traditional chemotherapy.
- Longitudinal studies suggest that patients can maintain remission with dasatinib for extended periods, supporting its use in long-term treatment strategies.
Such insights are pivotal for informing treatment guidelines and enhancing patient outcomes, helping healthcare professionals make educated decisions regarding the use of Sprycel in therapy.
Alternative Medications within the PBS Framework
In the Australian PBS (Pharmaceutical Benefits Scheme) framework, there are several alternative medications available for patients who may not tolerate Sprycel or when it is ineffective. Options include drugs like imatinib (Glivec), nilotinib (Tasigna), and bosutinib (Bosulif), which also target similar pathways in the treatment of blood cancers.
Sourcing these alternatives typically involves consultation with a healthcare professional and may consider patient-specific factors such as previous treatment responses.
Competition within this space ensures that patients have access to various options for effective leukaemia treatment, fostering a landscape where tailored therapeutic strategies can emerge based on patient needs and clinical responses.
Regulations Surrounding Sprycel
Understanding the regulations for Sprycel is crucial for patients and healthcare providers alike. This medication, containing dasatinib as its active ingredient, is classified as a prescription-only medication in Australia.
As a patient, being aware of its approval status can guide treatment decisions. Here’s a summary of significant points:
- Approved by the Australian Therapeutic Goods Administration (TGA) for chronic myeloid leukaemia (CML) and acute lymphoblastic leukaemia (ALL).
- Listed on the Pharmaceutical Benefits Scheme (PBS), making it accessible to many patients who meet certain criteria.
- Regular monitoring is required for side effects, especially blood disorders like neutropenia and thrombocytopenia.
Regulatory guidelines ensure Sprycel's efficacy while prioritising patient safety. Healthcare providers must strictly adhere to dosing instructions and monitor for potential drug interactions.
Frequently Asked Questions About Sprycel
Patients often have concerns and questions regarding their treatment with Sprycel. Addressing these questions can demystify the process and improve compliance.
Some typical inquiries include:
- What should I do if I miss a dose? If a dose is missed, take it as soon as remembered, but skip it if it’s close to the next scheduled dose. No doubling up.
- Can I purchase Sprycel without a prescription? It is generally not recommended to acquire Sprycel without a valid prescription to ensure proper usage and care.
- Are there any lifestyle changes I should consider while on Sprycel? Yes, maintaining a healthy diet, staying hydrated, and regular check-ups can aid in managing side effects effectively.
- What are common side effects? Patients may experience side effects like fatigue, nausea, and skin reactions. Reporting these to a healthcare provider is essential for support.
The FAQ section serves as a valuable resource for patients navigating their treatment protocols with Sprycel. Keeping lines of communication open with healthcare professionals enhances overall care.
Guidelines for Responsible Use of Sprycel According to Australian Health Standards
Sprycel is a powerful medication and using it responsibly is essential for maximising its benefits and minimising risks. Adhering to Australian health standards ensures that patients receive optimal care.
Key guidelines for responsible use include:
- Follow prescribed dosages. For adults with Ph+ CML, the standard dosage typically starts at 100 mg once daily.
- Regular monitoring is vital for detecting side effects early, especially blood-related issues.
- Store Sprycel at room temperature, away from moisture and light, keeping it in the original packaging until use.
Healthcare professionals should continuously educate patients about potential side effects and the importance of reporting these promptly.
Additionally, considerations for dosage adjustments may apply based on age and organ function. This attention to detail is vital for tailoring treatment to each individual.
Effective communication between patients and their healthcare teams fosters a supportive environment, aiding in successful treatment outcomes. The management of Sprycel should be informed and collaborative, ensuring both safety and efficacy.
Delivery Information for Sprycel in Major Australian Cities
| City | Region | Delivery Time |
|---|---|---|
| Sydney | New South Wales | 5–7 days |
| Melbourne | Victoria | 5–7 days |
| Brisbane | Queensland | 5–7 days |
| Perth | Western Australia | 5–7 days |
| Adelaide | South Australia | 5–7 days |
| Canberra | Australian Capital Territory | 5–7 days |
| Hobart | Tasmania | 5–9 days |
| Geelong | Victoria | 5–9 days |
| Newcastle | New South Wales | 5–9 days |
| Wollongong | New South Wales | 5–9 days |
| Cairns | Queensland | 5–9 days |
| Gold Coast | Queensland | 5–9 days |
| Sunshine Coast | Queensland | 5–9 days |
| Toowoomba | Queensland | 5–9 days |